 As part of the wider attempts to create smaller batteries that can be recharged for longer and which offer more power, researchers at the University of Cambridge, along with a team from the Beijing Institute of Technology, have developed what is described as a 'novel multifunctional sulphur electrode'.
As part of the wider attempts to create smaller batteries that can be recharged for longer and which offer more power, researchers at the University of Cambridge, along with a team from the Beijing Institute of Technology, have developed what is described as a 'novel multifunctional sulphur electrode'.
The device, which combines an energy storage unit and an electron/ion transfer network, is said to be 'extremely promising' and to address issues such as low efficiency and capacity degradation.
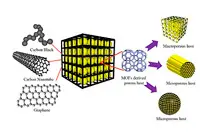
The approach uses a metal organic framework (MOF) as a 'template' to produce a conductive porous carbon cage. Sulphur within the cage acts as the host and each sulphur-carbon nanoparticle acts as an energy storage unit.
"Our carbon scaffold acts as a physical barrier to confine the active materials within its porous structure," said research scientist Kai Xi. "This leads to improved cycling stability and high efficiency."
However, the team has also brought graphene into play, 'wrapping' it around the sulphur electrode to speed the transport of electrons and ions.
According to Xi, the work provides a 'basic, but flexible, approach to both enhance the use of sulphur and to improve the cycle stability of batteries'. "Modification of the unit or its framework by doping or polymer coating could take the performance to a whole new level."
By integrating energy storage with an ion/electron framework, the team believes there is now the potential to make high performance energy storage systems that do not involve a structural change to a crystalline solid.
Author
Graham Pitcher
Source: www.newelectronics.co.uk
